Static Electricity
In this blog post I would like to explain the overall basics of what Static Electricity is and how it is made, as I currently understand it to be.
Nature of Charges
Protons have a positive charge, and Electrons have a negative charge. One charge of a Proton is equal to and opposite of one charge of a Electron, even though an Electron is only a fraction of the size and weight of a Proton. In a neutral state, an atom will have the exact same number of electrons that it has protons. An atom rarely ever gives up or takes in protons, since they are much heavier than electrons, therefore an atoms proton count always remains unchanged. However an atom will easily give up electrons, as well as take in extra electrons, which results in the atom to have less than or more than a normal amount of electrons.
In a natural, or neutral state, each atom in a body of matter will have the proper number of electrons in orbit around its nucleus to cancel out any opposing charge its nucleus has, allowing it to exist having a neutral (zero net) charge. Consequently, the whole body of matter composed of just neutral atoms will also be electrically neutral, since all of its atoms are electrically neutral. In this state, it is said to have a zero amount of (or no) charge. Electrons will neither leave nor enter the neutrally charged body should it come in contact with any other neutral bodies.
When in a natural, or neutral state, an atom has an equal number of electrons and protons. Because of this balance, the net negative charge of the electrons in orbit is exactly balanced by the net positive charge of the protons in the nucleus, making the atom electrically neutral.
However, if any quantity of electrons are removed from the neutral atoms of a body of matter, then those atoms will have more protons than they do electrons, which ultimately results in the whole body of matter to become Electrically Positive. Should the positively charged body come in contact with another body having a normal (or neutral) charge, or having a Negative charge (too many electrons) in comparison, or having a greater Positive charge (too few electrons) in comparison, an electric current will flow between them. Electrons will leave the more negative body and enter into the less negative (or positive) body. This electron flow will continue to flow between the two bodies until both bodies have an equal amount of electron charges.
Electrostatic Energy
When two bodies of matter have unequal charges and are in close proximity to each another, an electric force is exerted between them because of their unequal charges. However, since they are only near one another and are not actually in direct contact with each other then their charges cannot flow and equalize. The existence of such an electric force, where current cannot flow, is referred to as Static Electricity, which is also known as an electrostatic force.
One of the easiest and simplest ways to create a static charge between two bodies is by friction. When two pieces of matter are rubbed together, electrons can be swept off one material and pushed onto the other.
If the materials used are Good conductors, then it is actually quite difficult to obtain a detectable charge on either, since equalizing currents can flow very easily between the conducting materials. The conducting materials equalize the generated charges almost as fast as they are created.
A static charge is more easily created between Nonconducting materials. Since both materials are poor conductors, very little equalizing current can flow, and an electrostatic charge builds up. When the charge becomes great enough then current will flow between them regardless of the poor conductivity of the materials. These currents will usually cause visible sparks and produce a crackling sound.
For example, when a hard rubber rod is rubbed with fur, the rod will accumulate electrons given up by the fur. Another example is when someone rubs a filled balloon onto their hair, the skin of the rubber balloon will accumulate electrons given up by the hair, and an electrostatic charge will build up on the balloons surface. Another example is, while someone is wearing shoes, they drag their feet on the carpet as they walk, which causes their body to accumulate electrons given up by the carpet, and then when they touch a door knob (or another person even) the built up charge is discharged and a small electric spark is felt zapping from their finger tips.
Ions
Whenever an atom looses an electron, it now has less negative charge than it does positive charge (as compared to itself when it was in a natural neutral state), resulting in it to now be more positively charged. An atom becomes a positive ion whenever it loses an electron, and therefore it has an overall positive charge.
Whenever an atom gains an extra electron, it now has more negative charges than it does positive charge, resulting in it to now be more negatively charged (also as compared to itself when it is in a natural neutral state). Whenever an atom acquires an extra electron, it becomes a negative ion and has a negative charge.
Due to normal molecular activity within and between the atoms of materials, there are always ions present in any material. If the number of positive ions and negative ions are equal, the material is electrically neutral because the two equally opposing charges cancel each other out. When the number of positive ions exceeds the number of negative ions, the material is positively charged. The material is negatively charged whenever the negative ions outnumber the positive ions.
Since ions are actually just atoms without their normal number of electrons, it is the excess or the lack of electrons in a substance that determines its charge. In most solids, the transfer of charges is by movement of electrons rather than ions. The transfer of charges by ions will become more significant when considering electrical activity within liquids and gases.
Charged Bodies
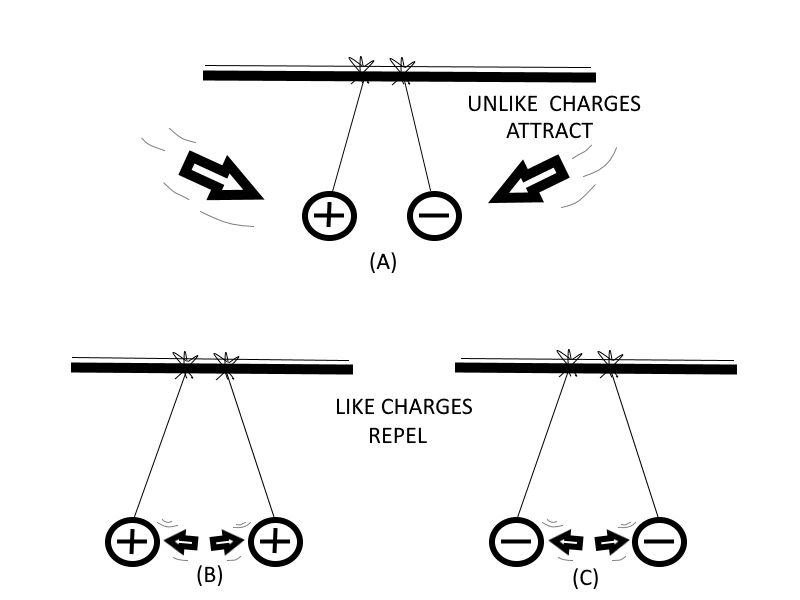
One of the fundamental laws of electricity is that Like Charges Repel Each Other and Unlike Charges Attract Each Other. A positive charge and negative charge, being unlike, tend to move toward each other.
In the atom, the negative electrons are drawn toward the positive protons in the nucleus. This attractive force is balanced by the electron's centrifugal force caused by its rapid rotation about the nucleus. As a result, the electrons remain in orbit and are not drawn into the nucleus.
Electrons repel each other because of their like negative charges, and protons repel each other because of their like positive charges.
| Reaction Between Charged Bodies |
Coulomb's Law of Charges
The relationship between attracting or repelling charged bodies was first discovered and written about by a French scientist named Charles A. Coulomb.
Coulomb's Law states : Charged bodies attract or repel each other with a force that is directly proportional to the product of their individual charges, and is inversely proportional to the square of the distance between them.
To phrase it in a different way, the amount of attracting or repelling force which acts between two electrically charged bodies in free space depends on two things:
- Their charges,
- The distance between them.
Coulomb's Law is also sometimes called the law of electric charges, or the law of electrostatic attraction.
Electric Fields
The space between and around charged bodies in which their influence is felt is called an Electric Field Of Force. In can exist in a vacuum, as well as in a medium (such as air, paper, glass, etc...). Electrostatic Fields and Dielectric Fields are other names commonly used to refer to this region of force.
Fields of force spread out in the space surrounding their point of origin and, in general, diminish in proportion to the square of the distance from their source.
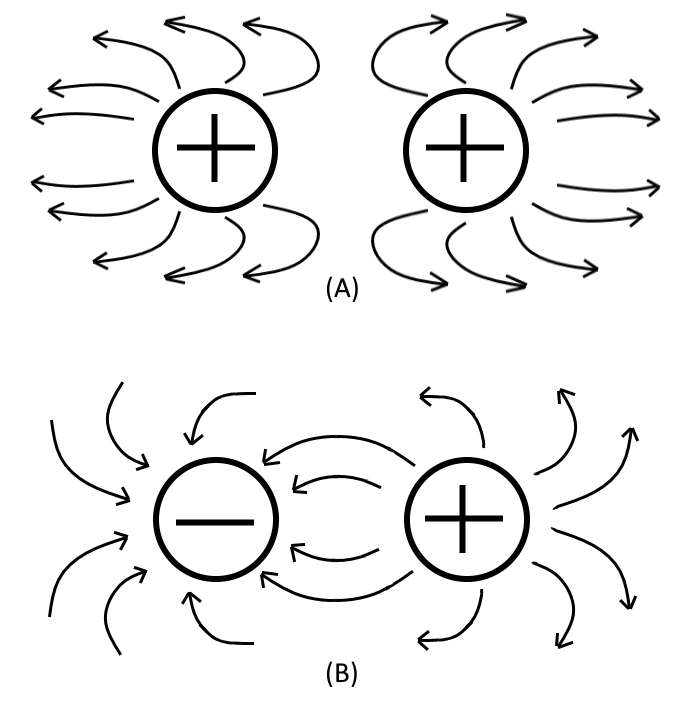
| Electrostatic Lines Of Force |
The field about a charged body is generally represented by lines which are referred to as Electrostatic Lines Of Force. These lines are imaginary and are used merely to visually represent the direction and strength of the field. To avoid confusion, using industry standards, the lines of force exerted by a positive charge are commonly always shown as leaving the charge, and for a negative charge they are shown entering the charge, as illustrated in the figure above. Also notice that (A) represents the repulsion of like-charged bodies and their associated fields. While (B) represents the attraction of unlike-charged bodies and their associated fields.
Final Thoughts
Thank you for reading, I hope you found this blog post educational and helpful in some way.