Understanding Batteries : Part 2
Continued from Batteries - Part 1...
BATTERIES
A battery is a voltage source that uses chemical action to produce a voltage. In many cases the term battery is applied to a single cell, such as a flashlight battery. In the case of a flashlight that uses a battery of 1.5 volts, the battery is a single cell. For batteries that provide more voltage than a single cell can provide, that battery is composed of multiple cells to provide the total amount required of it. If a cell is manufactured so that it provides 2 volts, then a 12 volt battery consisting of those cells would contain six 2 volt cells. Therefore, a battery is a device that combines one or more cells to provide a specific voltage output.
There are three ways to combine cells to form a battery:
- Series-Connected Cells : Cells connected in Series provide a higher voltage,
- Parallel-Connected Cells : Cells connected in Parallel provide a higher current capacity,
- Series-Parallel-Connected Cells (Complex) : Cells connected in Series-Parallel (Complex) provide both higher voltage and current.
In many cases, a battery powered device may require more electrical energy than one cell can provide. The device may require either a higher voltage or more current, and in some cases both. Under such conditions it is necessary to combine, or interconnect, a sufficient number of cells to meet the higher requirements.
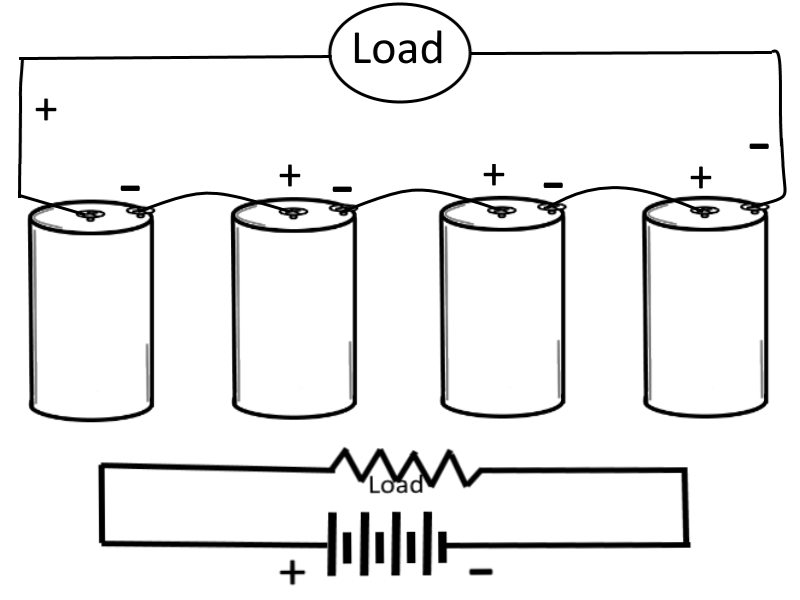
Series-Connected Cells
In this case (for this example), assume that a load requires a power supply of 6 volts and a current capacity of 1/8 ampere. Let's also assume that a single cell normally supplies a voltage of only 1.5 volts and a current of 1/8 ampere, therefore to meet the examples power requirement more than one cell is needed. To obtain the higher voltage, the cells are connected in series, where the negative terminal of one cell is connected to the positive terminal of the next cell, and so on and so forth until all of the inner cells are connected and only the outer terminals of the outer cells remain available to be connected to the load. In a schematic diagram, the load is shown by the resistance symbol and the battery is indicated by one long and one short line per cell.
In a series hookup, the negative electrode (cathode) of the first cell is connected to the positive electrode (anode) of the second cell, the negative electrode of the second to the positive of the third, etc. The positive electrode of the first cell and negative electrode of the last cell then serve as the terminals of the battery. In this way, the voltage is 1.5 volts for each cell in the series line. If we use four cells, then the output terminal voltage is 1.5 x 4, or 6 volts. When connected to the load, 1/8 ampere flows through the load and each cell of the battery. This is within the capacity of each cell. Therefore, only four series-connected cells are needed to supply this particular load.
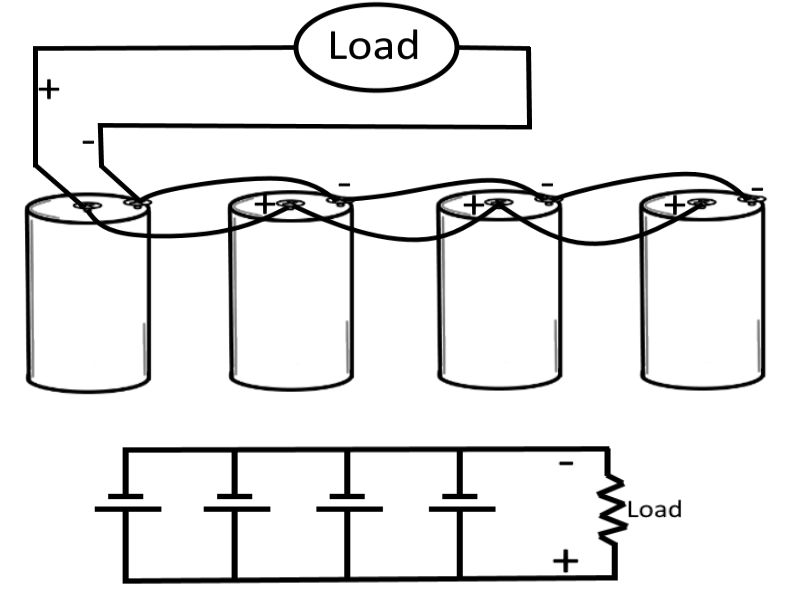
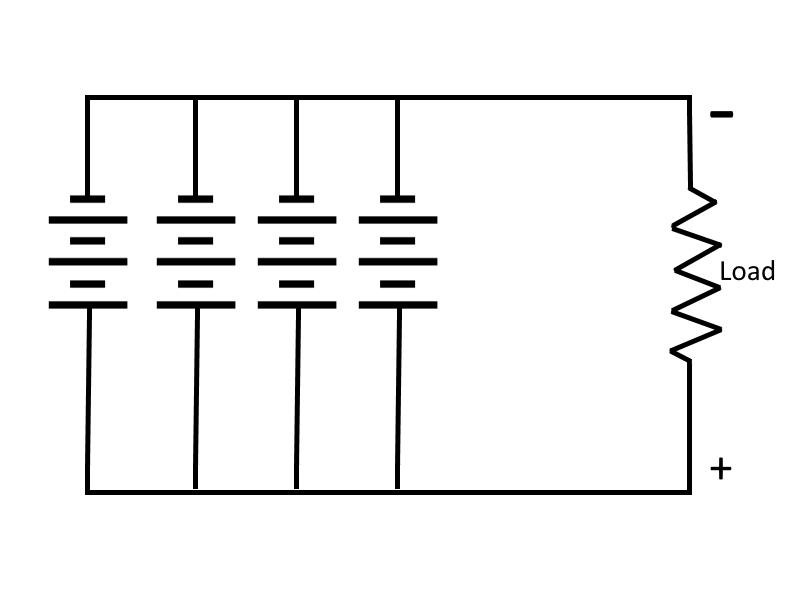
Parallel-Connected Cells
In this case (for this example), assume an electrical load requires only 1.5 volts, but will require 1/2 ampere of current. Let's also assume that a single cell will supply only 1/8 ampere. To meet this requirement, the cells are connected in parallel.
In a parallel connection, all positive cell electrodes are connected to one line, and all negative electrodes are connected to the other. No more than one cell is connected between the lines at any one point, so the voltage between the lines is the same as that of one cell, or 1.5 volts. However, each cell may contribute its maximum allowable current of 1/8 ampere to the line. There are four cells in this example, so the total line current is 1/8 x 4, or 1/2 ampere. In this case four cells in parallel have enough capacity to supply a load requiring 1/2 amperes at 1.5 volts.
Series-Parallel-Connected Cells
If a load requires more voltage and current than one cell can provide, then multiple cells must be connected in a more complex network (in a series-parallel-connected fashion).
For this case (for this example), assume that an electrical load requires 4.5 volts and 1/2 amperes of current. Let's also assume that a single cell will supply only 1.5 volts and 1/8 amperes.
To provide the required 4.5 volts, groups of three 1.5 volt cells are connected in series. To provide the required 1/2 amperes of current, four series groups are connected in parallel, each supplying 1/8 amperes of current.
Battery Construction
Some batteries are made from primary cells. When a primary cell battery is completely discharged, the entire battery must be discarded and replaced.
Secondary cell batteries are constructed using the various secondary cells as previously described above. The lead-acid battery and the nickel-cadmium battery are of the most common batteries in use today, so lets use them to explain battery construction.
For lead-acid batteries, the container houses the separate cells. Most containers are hard rubber, plastic, or some other material that is resistant to the electrolyte and mechanical shock (from being handled and moved around), and will withstand extreme temperatures. The container (the battery case) is vented through vent plugs to allow the gases that form within the cells to escape. The plates in the battery are the cathodes and anodes that were discussed earlier. The negative plate group is the cathode of the individual cells and the positive plate group is the anode. The plates are interlaced with a terminal attached to each plate group. The terminals of the individual cells are connected together by link connectors. The cells are connected in series in the battery and the positive terminal of one end cell becomes the positive terminal of the battery. The negative terminal of the opposite end cell becomes the negative terminal of the battery.
The terminals of a lead-acid battery are usually identified from one another by their size and markings. The positive terminal, marked (+) is sometimes colored red and is physically larger than the negative terminal, that is marked (-).
The individual cells of the lead-acid battery are not replaceable so in the event one cell fails then the entire battery must be replaced.
The nickel-cadmium battery is similar in construction to the lead-acid battery, with the exception that it has individual cells which can be replaced.
The construction of secondary cell batteries is so similar, that it is difficult to distinguish the type of battery simply by looking at it. The type of battery must be known to properly check and recharge the battery. Because of this, each battery should be manufactured to have a nameplate on it that gives a description of its type and electrical characteristics.
Capacity and Rating
The capacity of a battery is measured in Ampere-Hours (also known as amp-hours). The ampere-hour capacity is equal to the product of the current in amperes and the time in hours during which the battery will supply this amount of current. The ampere-hour capacity varies inversely with the discharge current.
For example, a 400 ampere-hour battery will deliver 400 amperes for 1 hour before it is fully discharged. Alternatively, that same 400 ampere-hour batter can also deliver 1 ampere for 400 hours before it is fully discharged.
Storage batteries are rated according to their rate of discharge and ampere-hour capacity. Most batteries are rated according to a 20-hour rate of discharge. That is, if a fully charged battery is completely discharged during a 20-hour period, it is discharged at the 20-hour rate. Thus, if a battery can deliver 20 amperes continuously for 20 hours, the battery has a rating of 20 amperes x 20 hours, or 400 ampere-hours. Therefore, the 20-hour rating is equal to the average current that a battery is capable of supplying without interruption for an interval of 20 hours.
All standard batteries deliver 100 percent of their available capacity if discharged in 20 hours or more, but they will deliver less than their available capacity if discharged at a faster rate. The faster they discharge, the less ampere-hour capacity they have.
The low-voltage limit, which is a value that is specified by the manufacturer, is the limit beyond which very little useful energy can be obtained from a battery. This low-voltage limit is normally a test used in battery shops to determine the condition and health of a battery.
Battery Charging
Simply by adding the active ingredient to the electrolyte of a discharged battery does not recharge the battery. Adding the active ingredient only increases the specific gravity of the electrolyte solution and does not convert the discharged plates back to their active materials, and therefore it does not bring the battery back to a charged condition.
A charging current must be passed through the battery to recharge it.
Batteries are usually charged in well vented battery recharging stations in mechanic garages, There are specific charging procedures that should be taken for the types of batteries to be charged.
The following types of charges may be given to a storage battery, depending upon the condition (health) of the battery:
- Initial charge : when a new battery is shipped dry, the plates are in an uncharged condition. After the electrolyte has been added, it is necessary to charge the battery, by giving the battery a long low-rated initial charge.
- Normal charge : a routine charge that is given in accordance with the nameplate data during ordinary cycle of operation to restore the battery to its charged condition.
- Equalizing charge : a special extended normal charge that is given periodically to batteries as part of a maintenance routine. It insures that all the sulfate is driven from the plates and that all the cells are restored to a maximum specific gravity. Equalizing charge is continued until the specific gravity of all cells show no change for a 4-hour period.
- Floating charge : the charging rate is determined by the battery voltage rather than by a definite current value. The floating charge is used to keep a battery at full charge while the battery is idle or in light duty. It is sometimes referred to as a Trickle Charge and is accomplished with low current.
- Fast charge : used when a battery must be recharged in the shortest possible time. The charge starts at a much higher rate than is normally used for charging. It should be used only in an emergency, as this type of rapid forceful charging technique may be harmful to the battery and could damage it beyond repair.
The charge must be continued until the battery is fully charged. Frequent readings of specific gravity should be taken during the charge, and compared with the readings taken before the battery was placed on the charger. Automatic battery chargers may do something similar by taking periodic readings of the batteries voltage and ampere ratings at different times and comparing them to previous readings and possibly even to internal lookup charts that may be contained within the memory circuits of that automatic battery charger.
When a battery is being charged (charged, recharged, etc...), a portion of the energy breaks down the water in the electrolyte. Hydrogen is released at the negative plates and oxygen at the positive plates. These gases bubble up through the electrolyte and collect in the air space at the top of the cell. If violent gassing occurs when the battery is first placed on charge, the charging rate is too high. If the rate is not too high, steady gassing develops as the charging proceeds, and can be observed to indicate when the battery is nearing a fully charge condition.
Safety
Battery cells are used to generate electricity by chemical means, meaning that they contain both Chemicals and Electricity. The chemicals used in batteries are often very poisonous and acidic in nature, so take care to not get any on your skin, and seek immediate medical attention if ingested. Electricity of any amount should be respected and treated with utmost caution and care. Regardless of the amount of Voltage, as little as 0.3 Amps of electrical current passing through the chest of a persons body is all it takes to cause that persons heart to stop, and most batteries can generate much more power than that.
Electricity should not be feared, but it should always be respected and handled with caution.
If the anode and cathode of a battery cell are connected together without a load, a Short Circuit condition exists, which can be very dangerous. This condition cause excessive heat, pressure, and current flow which may cause serious damage to the cell or be a safety hazard to equipment or personnel. The excessive heat may be high enough to cause the battery (and/or cell) itself (or the material of any surrounding substance that is in physical contact with the battery and/or cell) to catch fire. Extra care should be taken to never short a mercury cell, as shorted mercury cells have been known to not only catch fire but to also explode with considerable force.
Lithium cells contain toxic materials that are under high pressure. Do not puncture, recharge, short circuit, expose to excessively high temperatures, or incinerate lithium cells. Lithium cells should be disposed of properly, and therefore they should not simply be thrown into a trashcan. If you have lithium cells and wish to dispose of them, simply take them to your local fire department and allow them to properly dispose of the lithium cells for you.
When connecting cells in series, connect alternate terminals together (- to +, - to +, etc..), always have two remaining terminals that are used for connection to the load only. Do not connect the two remaining terminals together as this causes a short across the battery and would not only quickly discharge the cells but could cause some types of cells to catch fire and/or explode.
A mixture of hydrogen and air can be dangerously explosive. No smoking, electric sparks, or open flames should be permitted near batteries while they are being charged or recharged.
Final Thoughts
Thank you for reading, I hope you found this blog post educational and helpful in some way.